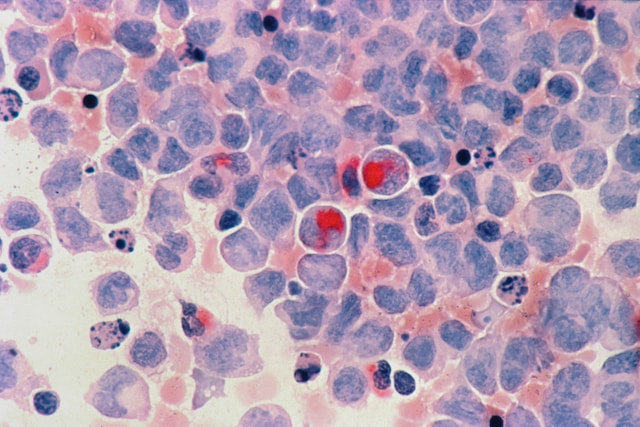
Scientists Discover Body’s ‘Kill Switch’ Capable of Destroying Cancer Cells
Researchers at have identified a ‘kill switch’ within the body that prompts cancer cells to self-destruct, offering new treatment avenues.

These CD95 receptors are situated on the surface of cells and, when activated, trigger a cascade of molecular events leading to cell death. While their role in maintaining cellular balance has been recognized, their full potential in cancer therapy is now coming to light. “Previous efforts to target this receptor have been unsuccessful. But now that we’ve identified this epitope (target), there could be a therapeutic path forward to target Fas in tumors,” stated Jogender Tushir-Singh, senior author of the study and associate professor in the Department of Medical Microbiology and Immunology.
Currently, standard cancer treatments encompass surgery, chemotherapy, and radiotherapy. Additionally, immune-based therapies, such as CAR (chimeric antigen receptor) T-cell therapy, have been employed, though their effectiveness is limited to specific cancer types. “Despite being decently successful in liquid tumors, such as leukemia spectrum cancers, long-term remission remains the biggest challenge for CAR T-cell therapies,” Tushir-Singh shared.

The study’s findings suggest that targeting the Fas epitope could overcome therapeutic resistance by inducing programmed cell death. This approach not only holds promise for enhancing the efficacy of existing immunotherapies but also for extending their benefits to solid tumors. “It is evident that the success of CAR-T therapy relies on off-target killing by Fas,” Tushir-Singh noted. He further emphasized the importance of screening potential cancer patients for the presence of Fas on their tumors before undergoing CAR-T therapy.
This discovery offers renewed hope in the fight against cancer, potentially leading to more effective treatments and improved patient outcomes.